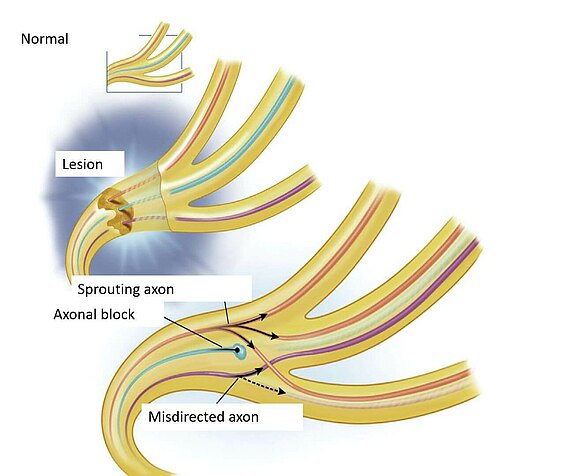
In principle, nerve cells, especially in younger patients, have a pronounced potential for regeneration [1]. However, if there is a partial or complete interruption of continuity in the area of the peripheral extensions of the axons due to an accident or tumor disease, their function may be partially or completely impaired. Partial or complete facial paralysis is present. If the nerve ends are close to each other (or have been adapted by a microsurgical repair) a complete healing and a complete functional regression may occur [2]. If there is a separation or local damage (e.g. pressure damage due to strong swelling of the nerve in the part of its bony course in the temporal bone in the context of Bell's palsy with subsequent reduced blood flow), the damage is " standardized" according to the principle of the "Wallerian degeneration" (see from pathophysiology) [3]. Proximal to the lesion (towards the cell body in the brain), the nerve continues to "live", while the distal part dies and becomes dysfunctional with the corresponding dysfunctions of the affected musculature.
The distal part, however, remains as a structure and can serve as a guide for axons sprouting from the living nerve part, if they can reach it. This is possible if the sheath structure (the myelin sheath) is still intact, or the severed nerve is reattached by a microsurgical nerve suture. The nerve fibers usually need at least one month to be able to skip a nerve suture. Here it is important that continuity is restored, otherwise the axons cannot grow into each other. The successful axons take the distal, "dead" part of the nerve path in their growth as a guide to reach their successful organ, the facial muscle, again. A loss of 75-80% of the nerve fibres is expected along this route, i.e. only about 20-25% of the axons present in the nerve part near the brain actually reach the target organ, the muscle[4, 5]. Under the best conditions, this is assumed to be about 1mm growth distance per day[6].
Calculation example: If the site of damage is located in the petrous bone, e.g. as suspected in Bell's paresis[3], about 10 cm away from the facial muscle in the midface (e.g. upper lip lifter), 1 mm growth per day theoretically takes 100 days (i.e. about three months) until the muscle can be activated again.
If after a period of half a year (6 months) no muscle activity or signs of improvement occur, it can be assumed that the mechanism of "reinnervation", i.e. the re-response of the muscle by the newly sprouting nerve has not worked and remains unlikely in the future (see also prognosis and timing, time-is-muscle).
Sometimes, the healing nerve fiber ends are exchanged during ingrowth. This is similar to a power cable, which consists of several individual wires, whereby the blue line connects with the green line and the system no longer functions properly. A so-called synkinesis develops (see here also sykinesis): In facial expressions, unwanted, disturbing movements of unintentionally controlled muscle groups occur. If the nerve to the masseter muscle (N. massetericus) is repositioned as part of a reconstruction, the brain must learn that the nerve that formerly controlled a chewing function now innervates mimetic muscles. The ability to "relearn" is called neuronal plasticity. Of course, the process involves a lot of training and rehabilitation on the part of the patient, but the brain still has this ability at later ages [7].
By adapting its structures and functions, the human nervous system in particular is able to react in an adapted manner to externally applied (extrinsic) and internally based (intrinsic) stimuli.[8] This ability is called neuronal plasticity. Stimuli for this are, for example, nerve lesions. Already within the first 48 hours after the lesion an activation of different neuronal networks occurs, which tries to compensate for the initial damage.[9] In the further course of the lesion new nervous connections are formed by so-called "synaptic plasticity". "Synaptic plasticity" is based on the principle of "long-term potentiation". This means that when a nerve cell (neuron) is stimulated, it releases more messenger substances (neurotransmitters) and is consequently more easily excitable. This results in a permanent reduction or strengthening of nerve connections. Repeated practice of a corresponding movement can have a positive effect on "synaptic plasticity"[10] Another example of neuronal plasticity is adult neurogenesis, i.e. the formation of new nerve cells even in adulthood. It is currently assumed that the olfactory bulb (Bulbus olfactorius) and the hippocampus are involved[11].
A further field of neuronal plasticity is functional restructuring, which is based primarily on the concepts of equivalence and vicarication. The principle of equivalence covers the compensation of damaged areas of the brain by the opposite side, especially in younger patients[12] In contrast, vicarication involves the takeover of failed functions by brain areas with originally different tasks. It should be emphasized that both concepts only convey a basic idea without correctly mapping neuronal plasticity in detail. Furthermore, the so-called "diaschisis" summarizes the approach that an injury in a certain brain section can also lead to an impairment of areas far away from the lesion.[13] According to Monakow, the classical variant describes, for example, the reduced blood supply of an area in the course of a brain infarction, although another area should be affected by the infarction. The functional dia-schisis, which only detects the loss of blood flow in one area of the brain when another area is activated, is to be distinguished from this. Furthermore, neuronal plasticity apparently takes place more slowly in areas of higher brain functions[14].
In facial nerve palsy patients, synkinesia in particular shows an overall weaker activation of the motor cortex, i.e. the area mainly responsible for voluntary motor activity.[15] In addition, motor areas in healthy individuals are generally equally stressed on both sides during facial movements. In patients with facial nerve palsy, a one-sided dominance in the use of these areas can be observed. This could also be related to an impairment of neuronal plasticity.
Sources:
[01] Yeo S-W, Lee D-H, Jun B-C, Chang K-H, Park Y-S. Analysis of prognostic factors in Bell's palsy and Ramsay Hunt syndrome. Auris Nasus Larynx 2007; 34(2):159–64.
[02] M F G, M M, S H, Khan WS. Peripheral nerve injury: principles for repair and regeneration. Open Orthop J 2014; 8:199–203.
[03] Glass GE, Tzafetta K. Bell's palsy: a summary of current evidence and referral algorithm. Fam Pract 2014; 31(6):631–42.
[04] Mackinnon SE, Dellon AL, Hunter DA. Histological assessment of the effects of the distal nerve in determining regeneration across a nerve graft. Microsurgery 1988; 9(1):46–51.
[05] Frey M, Happak W, Girsch W, Bittner RE, Gruber H. Histomorphometric studies in patients with facial palsy treated by functional muscle transplantation: new aspects for the surgical concept. Ann Plast Surg 1991; 26(4):370–9.
[06] Buchthal F, Kühl V. Nerve conduction, tactile sensibility, and the electromyogram after suture or compression of peripheral nerve: a longitudinal study in man. J Neurol Neurosurg Psychiatry 1979; 42(5):436–51.
[07] Buendia J, Loayza FR, Luis EO, Celorrio M, Pastor MA, Hontanilla B. Functional and anatomical basis for brain plasticity in facial palsy rehabilitation using the masseteric nerve. J Plast Reconstr Aesthet Surg 2016; 69(3):417–26.
[08] Mateos-Aparicio P, Rodríguez-Moreno A. The Impact of Studying Brain Plasticity. Front Cell Neurosci. 2019 Feb 27;13:66. doi: 10.3389/fncel.2019.00066.
[09] Sophie Su Y, Veeravagu A, Grant G. Neuroplasticity after Traumatic Brain Injury. In: Laskowitz D, Grant G, edi-tors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 8. PMID: 26583189.
[10] Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013 Nov;37(9 Pt B):2243-57. doi: 10.1016/j.neubiorev.2013.04.005. Epub 2013 Apr 25. PMID: 23623982.
[11] La Rosa C, Parolisi R, Bonfanti L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front Neurosci. 2020 Feb 4;14:75. doi: 10.3389/fnins.2020.00075. PMID: 32116519; PMCID: PMC7010851.
[12] Finger S. Chapter 51: recovery of function: redundancy and vicariation theories. Handb Clin Neurol. 2010;95:833-41. doi: 10.1016/S0072-9752(08)02151-9. PMID: 19892154.
[13] Finger S. Chapter 51: recovery of function: redundancy and vicariation theories. Handb Clin Neurol. 2010;95:833-41. doi: 10.1016/S0072-9752(08)02151-9. PMID: 19892154.
[14] Klingner CM, Volk GF, Brodoehl S, Burmeister HP, Witte OW, Guntinas-Lichius O. Time course of cortical plastic-ity after facial nerve palsy: a single-case study. Neurorehabil Neural Repair. 2012 Feb;26(2):197-203. doi: 10.1177/1545968311418674. Epub 2011 Aug 29. PMID: 21875890.
[15] Wang Y, Wang WW, Hua XY, Liu HQ, Ding W. Patterns of cortical reorganization in facial synkinesis: a task func-tional magnetic resonance imaging study. Neural Regen Res. 2018 Sep;13(9):1637-1642. doi: 10.4103/1673-5374.235304. PMID: 30127126; PMCID: PMC6126138.